Tips & Techniques in Pre-analytics Volume 1: Blood analysis in companion animals
Tips & Techniques in Pre-analytics Volume 1: Blood analysis in companion animals
Language:
| Order number | 04.1954.001 |
|---|---|
| Product description | S-Monovette® Lithium heparin gel+ LH, preparation: Lithium heparin gel+, 4.9 ml, membrane screw cap, cap orange, colour code EU, (LxØ) without cap: 90 x 13 mm, with paper label, label/print: brown, 50 piece(s)/case, sterile |
| Type of collection | venous |
|---|---|
| Type of preparation | Lithium heparin gel+ |
| Preparation concentration | 25 IU/ml blood |
| Colour code | EU |
| Label/ Print | with paper label |
| Colour of print/label | brown |
| Graduation | fill mark |
| Cap | membrane screw cap |
| Closure type | screw cap |
| Recommended centrifugation | 2500 x g |
| Centrifugation temperature | 20 |
| Centrifugation time | 7 Min |
| Liquid-tight | yes |
| Connection | membrane screw cap |
| Sample volume | 4.9 ml |
|---|---|
| Outer diameter of cap | 16 mm |
| Diameter | 13 mm |
| Length including cap | 106 mm |
| Length excluding cap | 90 mm |
| Length including cap and plunger | 127 mm |
| Colour of product | transparent |
|---|---|
| Closure material | High Density Polyethylene (HD-PE) |
| Colour of cap | orange |
| Membrane material | rubber |
| Tube material | Polypropylene (PP) |
| Piston material | High Density Polyethylene (HD-PE) |
| Piston plunger material | Polystyrene (PS) |
| Satisfies the requirement | IATA, ADR |
|---|---|
| Product category | In vitrodiagnostic device, CE |
| CE certified | CE |
| Purity standard | sterile |
| Sterilisation | Electron irradiation |
| Batched | yes |
| Minimum order qty. | 500 |
|---|---|
| Type of smallest subpackaging | case |
| Piece(s) / inner box | 50 |
| Piece (s) / outer case | 500 |
| Piece(s) / pallet | 36000 |
| Depth of box | 171 mm |
| Width of box | 130 mm |
| Height of box | 73 mm |
| Depth of case | 388 mm |
| Width of case | 358 mm |
| Height of case | 150 mm |
| Case volume | 0.0208 cbm |
| Weight of product | 0.0057 kg |
| Weight of case | 3.63 kg |
| EAN of inner box | 4038917523158 |
| EAN case | 4038917523141 |





















For ESR determination using the S-Sedivette® a sedimentation pipette is not required. Blood collection, transport and measurement can be safely carried out in the enclosed blood collection tube, the S-Sedivette®.
The screw cap enables a gentle opening of the tubes and minimises the aerosol effect which occurs when using stoppers.
Yes, the neutral S-Monovette® is free from additives and is available in various different designs.
Yes, the S-Monovette® meets the requirements of the IATA guidelines and is impervious to fluids at a pressure difference of 0.95 kPa.
We recommend storage at room temperature.
The S-Monovette® is made entirely from plastic. It is therefore particularly unbreakable and ideally suited for transport. The main components are the tube made from polypropylene (PP), the membrane screw cap made from polyethylene (PE) and natural rubber and the plungers made from polyethylene (PE).
The S-Monovette® is packed in a cardboard box filled with 50 items as standard. This enables low levels of waste generation and simple disposal.
The membrane is made from natural rubber and is embedded lower down in the screw cap in order to prevent direct contact with the puncture site. The result is improved infection protection, allowing the user to work safely with the S-Monovette®.
An expiry date is necessary in order to rule out any influence caused by the ageing process and in order to ensure a consistently high sample quality. This is indicated as the month and year and can be found on both the product and the packaging.
The S-Monovette® is classified as an in vitro diagnostic tool and is CE marked. The entire quality management system is certified in accordance with EN ISO 13485.
The age of the S-Monovette® has no impact on the filling volume. The S-Monovette® is designed so that 100% of the nominal volume can always be obtained.
Yes, the paediatric S-Monovette® is available in volumes 1.1 ml–1.4 ml, depending on the preparation.
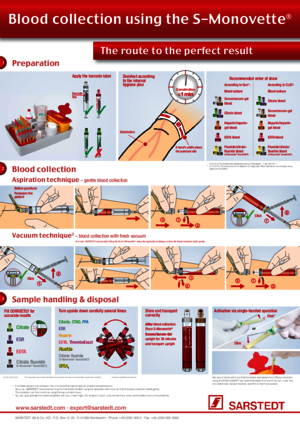
The patient should sit for at least 10 minutes before blood collection, stasis time, puncture, collection order, correct filling (citrate, EDTA), mixing the sample, storage and transport conditions (e.g. temperature), blood collection: selection of method – aspiration or vacuum.
Depending on the degree of haemolysis, haemolytic samples distort or prevent the determination of analytes and the blood has to be collected again. Here are a few example of parameters which can be affected: LDH, K, AST (GOT), ALT (GPT), troponin, ß-HCG, glucose, CK, PT, aPTT, D-dimer.
Here are some of the most important influencing factors for preventing haemolytic samples: avoid vigorous shaking, prevent heavy vibrations during transport of the blood sample, when collecting blood in particular from inserted catheters only collect blood by means of aspiration, use needles with large lumens.
A slower, more gentle blood flow can be achieved by choosing aspiration as a blood collection technique. This also causes the smallest possible effect on the platelets.
Relative centrifugal force (RCF) is the ratio between the number of rotations and the radius of the centrifuge. The values can be calculated from one another using the formula: RCF = 1.118*10-6*r*n2. r = radius of the centrifuge, n = number of rotations (= rotations/min), g = 9.81 m/s2 (= gravity), RCF = relative centrifugal force.
The inserts in the fixed-angle rotor are at a fixed angle to the axis of rotation (often 30°) and remain at this angle during centrifugation. In contrast to this, the inserts in the swing-out rotor move into a position perpendicular to the axis of rotation at the start of centrifugation and remain there. This is the only way to achieve an optimal gel separating layer.
Carry out blood collection using the aspiration technique. An optimal solution is to collect blood using a Safety-Multifly® needle and S-Monovette®.
Yes, very much so. Blood should be collected from the patient when he or she is in a sitting or lying position. Before blood collection, the patient should rest sit for 10 minutes and avoid physical activity.
The time of blood collection plays an important role for many laboratory parameters, so blood should normally be collected in the morning between 7 a.m. and 9 a.m. from patients with an empty stomach (drinking water is permitted).
You can find more information on our website in the Service section, under Publications.
Haemolysis occurs as a result of damage to the cell membrane of erythrocytes. The red haemoglobin (main component of red blood cells) which is then released can disrupt the analysis of various different parameters, depending on the amount released.
Underfilling can cause an incorrect ratio between the citrate presented and the blood and can therefore lead to incorrect results.
The S-Monovette® Glucose must be carefully inverted in order to ensure optimal distribution of the preparation in the blood.
The shelf life of the S-Monovette® Glucose is 12 months.
The S-Monovette® Glucose is prepared with fluoride and EDTA.
The S-Monovette® GlucoEXACT is prepared with citrate and fluoride.
The two S-Monovettes differ in terms of their preparation and, as a result of this, in terms of the start of glycolysis inhibition. Glycolysis inhibition occurs in the S-Monovette® GlucoEXACT, which is filled with blood, immediately after the blood is collected. In contrast, the inhibition in the S-Monovette® which is only prepared with fluoride starts after approximately two hours.
A gel preparation should be used when a stable barrier between the blood clot and the serum and between the blood cells and the plasma is needed for transport and storage. The gel's thickness means that during centrifugation it forms a separating layer between the blood clot and the serum or the blood cells and the plasma, providing a stable barrier for transport and strorage.
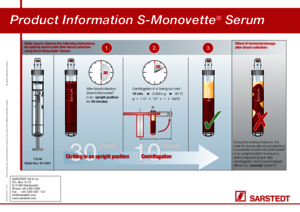
For optimal serum quality, we recommend that the S-Monovette® Serum/Serum Gel be stored standing upright for at least 30 minutes before being centrifuged.
Once the gel barrier has formed, further centrifugation is not recommended as the barrier effect of the gel separating layer would no longer be guaranteed.
Yes, there is a silicate on the granulate beads in the S-Monovettes.
The gel in the S-Monovette® is made from a polyacrylic ester which, due to its thickness, forms a stable separating layer during centrifugation.
In order to achieve an evenly formed separating layer, centrifugation must be carried out in a centrifuge with a swing-out rotor. The S-Monovette® Serum-Gel is to be centrifuged at 2500 xg for 10 minutes and the S-Monovette® Plasma-Gel at 2500 xg for 15 minutes or 3000 xg for 10 minutes, both at room temperature.
The plastic granulate is coated with the respective preparation and supports the mixing process between the blood and the preparation.
The S-Monovette® Metal Analysis (product number 01.1604.400) in combination with the Safety-S-Monovette® Needle for Metal Analysis (product number 85.1162.600) is recommended for the determination of heavy metals. The S-Monovette® Serum and S-Monovette® Heparin have not been evaluated for the determination of heavy metals.
The shelf life of the S-Monovette® Serum is 36 months.
The shelf life of the S-Monovette® Lithium-Heparin is 36 months.
An exact filling volume and therefore a correct mixing ratio is recommended to avoid incorrect measurements due to underfilling.
The S-Monovette® Citrate is prepared with trisodium citrate solution which makes up 10% of the nominal volume. This achieves the ratio which is important for clotting diagnostics – 1 part citrate to 9 parts blood.
The recommended centrifugation for the S-Monovette® Citrate is 1800 x g for 10 minutes at room temperature.
The S-Monovette® Citrate should be carefully inverted 8–10 times after the blood has been collected.
When taking blood using a Safety-Multifly® needle, blood remains in the tube (known as dead volume), reducing the volume of blood in the first S-Monovette®. If only one S-Monovette® Citrate is to be collected at first or in total, carrying out the preceding collection using an empty tube is recommended to avoid underfilling.
The shelf life of the S-Monovette® Citrate is 15 months.
Studies have shown a significantly improved recovery rate in EDTA Gel S-Monovettes compared to EDTA S-Monovettes.
EDTA K3 stands for ethylenediaminetetraacetate tripotassium. It binds calcium and prevents clotting. The blood sample remains liquid.
The S-Monovette® EDTA is prepared with K3 EDTA.
The shelf life of the S-Monovette® EDTA is 18 months.
The S-Monovette® ThromboExact is recommended for these patients.
The S-Monovette® EDTA must be carefully inverted in order to ensure optimal distribution of the preparation in the blood.
The S-Monovette® GlucoEXACT is recommended.
The S-Monovette® Metal Analysis (product number 01.1604.400) in combination with the Safety-Needle for Metal Analysis (product number 85.1162.600) is recommended for the determination of heavy metals. The normal S-Monovette® Serum and S-Monovette® Heparin have not been evaluated for the determination of heavy metals.
Yes, the S-Monovette® ThromboExact has been specially developed for anticoagulant-induced pseudothrombocytopenia. If the thrombocyte count is low in EDTA blood, the S-Monovette® ThromboExact can be used to find out whether the thrombocyte count is a false low result and the patient is experiencing anticoagulant-induced pseudothrombocytopenia or whether the thrombocyte count really is low.
The following routine clotting parameters can be measured using the S-Monovette® CTAD: aPTT, PTT (Quick), fibrinogen, thrombin time, AT3 and Protein C. Individual tests must be carried out for all further parameters.
The S-Monovette® Hirudin which was developed for the Multiplate device by Roche (formerly Verum Diagnostica) and the S-Monovette® PFA100 which was designed for the PFA100/PFA200 device by Siemens are recommended here. Both S-Monovettes can be used for the special thrombocyte function diagnostics.
The S-Monovette® Homocysteine HCY-Z-Gel is recommended, which stabilises the HCY concentration up to 96 hours after the blood is collected.
We would like to show you additional content which is hosted by a third party. For this purpose we need your consent to set cookies: