
With the EU Declarations of Conformity of our medical devices, we confirm compliance with the essential requirements of the European Council Medical Device Directive 93/42/EEC (MDD) and the German Medical Devices Law.
With the EU Declarations of Conformity of our in-vitro diagnostic devices, we confirm compliance with the essential requirements of the Council Directive 98/79/EC on in-vitro diagnostic devices (IVDD) and the German Medical Devices Act.
The EU Declaration of Conformity is a mandatory prerequisite for CE marking and placing the corresponding product on the market in the European Economic Area.
Category:
Hits:


Urine Transfer Straw 11.1240.101

Urine Transfer Straw 11.1240.100
Urine Transfer Straw 11.1240.100
Downloads:
Container with screw cap 100 ml

MDR SARSTEDT SAHARA-TSC

MDR Navel clamp

MDR safety lancet

MDR S-Monovette® safety needles / needles

MDR Safety-Multifly® Needles/Multifly® Needles

SAHARA 4

SAHARA-III / -III MAXITHERM

Container 70 ml with cap

SARSTEDT Sediplus ® S 2000 NX

Urine Cups 125ml

Ventilator for Blood Gas Monovette®

Blood sedimentation racks and pipettes

Coombs tube
Sample Cups for Analysing Systems
Blood Gas Capillaries and Accessories

Faeces Tubes

Pipette tips

Urine Container

Archiving Cap

Adapter Sleeve and Needle Holder

Prepared Micro Tubes

UriSet 24

Prepared Tubes

Tube Segment Opener

Formalin System

Transfer Pipette

Salivette®

Multivette® 600

Screw Cap Micro Tubes

Minivette® POCT

Microvette®

Micro Tubes

Tubes

Monovette® Urine

PCR Tubes

S-Monovette®, Monovette®, S-Sedivette® (sterile)
S-Monovette®, Monovette®, S-Sedivette® (sterile)
Downloads:

tournistrech® / tournistrip® disposable tourniquet, latex-free
tournistrech® / tournistrip® disposable tourniquet, latex-free
Downloads:

Tourniquet (latex-free and not)

Membrane-Adapter (sterile)

Multi-Adapter (sterile)

Safety-Multifly® BCF Set

Bloodculture adaptet (sterile)

V-Monovette® Urin (steril)

Urine-Monovette® (sterile)

Urine container and lid (sterile)

Medicine cup

V-Monovette® (sterile)

Safety-Heel®

Feces container (sterile)

Formalin System and accessories (sterile)

Sample Distribution System PVS

Sample Aliquoting System AL-Flex

Bulk Sorter HCTS2000 MK2

Sample Distribution System HSS

Bulk Sorter BL 1200

Sample Distribution System Flex

Recapper RC 1200 / Recapper 1200 S

Decapper DC 1200

Micro Tubes for IVD purposes (sterile)

Tubes for IVD purposes and accessories (sterile)
Tubes for IVD purposes and accessories (sterile)
Downloads:

Analyzer Cups (sterile)

Tubes with Saline Solution (sterile)

Transport swabs (sterile)

Pipette tips (sterile)

Transfer pipettes (sterile)

Swabs, no medium

Swabs, gel medium
Centrifuge, SC 2700

Luer cannula, (ØxL): 25G x 16 mm, orange

Disposable syringe, for miniPERM® bioreactor, 50 ml, Luer lock
Disposable syringe, for miniPERM® bioreactor, 50 ml, Luer lock
Downloads:

Disposable syringe, for miniPERM® bioreactor, 2 ml, Luer

CryoPure Tubes (sterile)

Urine bag sterile

Secretion bag
UroStretch

Enclosed Urine Drainage Systems
Enclosed Urine Drainage Systems
Downloads:
Tempus600 Quantit TQ004
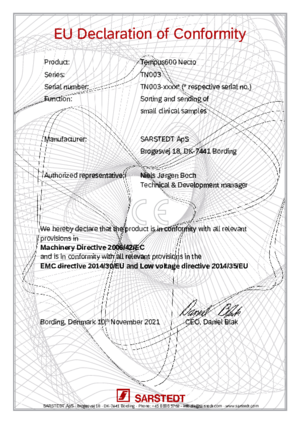
Tempus600 Necto
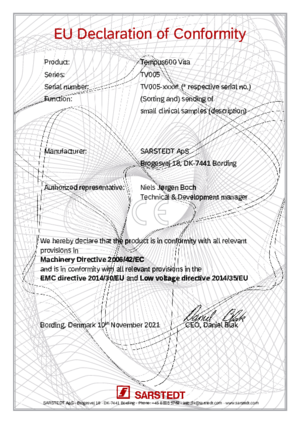
Tempus600 Vita
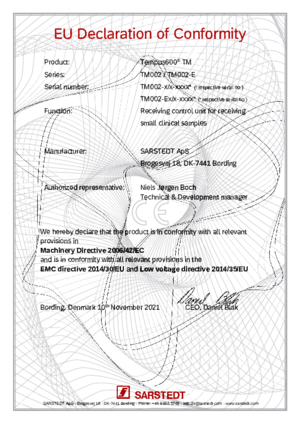
Tempus600® TM
No matching files found.