Sediplus® System Solutions
Sediplus® System Solutions
Language:
| Order number | 47.410 |
|---|---|
| Product description | Sample tube, nominal volume: 2 ml, cap violet, preparation: Citrate, 4NC, screw cap, (LxØ): 66 x 11.5 mm, flat base, with print, 100 piece(s)/bag |
| Type of collection | venous |
|---|---|
| Type of preparation | Citrate, 4NC |
| Colour code | EU |
| Label/ Print | with print |
| Colour of print/label | violet |
| Graduation | yes |
| Closure type | screw cap |
| Suitable for | Sediplus® |
| Base shape | flat base |
| Sample volume | 2 ml |
|---|---|
| Diameter | 11.5 mm |
| Length of product | 66 mm |
| Length including cap | 67 mm |
| Length excluding cap | 66 mm |
| Colour of product | transparent |
|---|---|
| Closure material | High Density Polyethylene (HD-PE) |
| Colour of cap | violet |
| Tube material | Polypropylene (PP) |
| Satisfies the requirement | IATA, ADR |
|---|---|
| Product category | In vitrodiagnostic device, CE |
| CE certified | CE |
| Batched | yes |
| Minimum order qty. | 500 |
|---|---|
| Type of smallest subpackaging | bag |
| Piece(s) / inner box | 100 |
| Piece (s) / outer case | 500 |
| Piece(s) / pallet | 48000 |
| Depth of case | 348 mm |
| Width of case | 278 mm |
| Height of case | 140 mm |
| Case volume | 0.0135 cbm |
| Weight of product | 0.0027 kg |
| Weight of case | 1.64 kg |
| EAN of inner box | 4038917081443 |
| EAN case | 4038917000536 |

Yes, the Sediplus® S 2000 NX can be integrated through an interface.
No, each measuring station can be filled individually / independently of time.
Yes, the ESR can be determined automatically with the Sediplus® S 2000 NX (40 measuring stations).
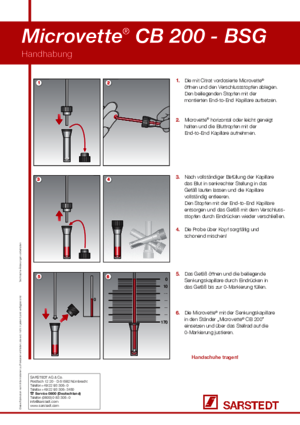
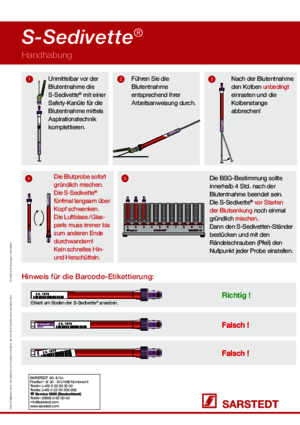
For blood sedimentation, the S-Sedivette® should always be carefully mixed after blood collection. According to the CLSI standard, the measurement must be started no later than 4 hours after blood collection. The first reading of the blood sedimentation rate (ESR) should take place after 60 minutes.
The S-Sedivette® is prepared with trisodium citrate solution, which is 20% of the nominal volume. This ensures the important ratio for blood sedimentation of 1 part citrate + 4 parts blood.
The S-Sedivette® has a shelf life of 15 months.
Yes, the S-Sedivette® must be mixed thoroughly immediately after blood collection: invert the S-Sedivette® slowly five times. The air bubble / glass bead must always travel to the other end. Do not shake it back and forth quickly. For safety and to save time, we recommend the special Sarmix® M 2000 mixers.
The S-Sedivette® was developed and evaluated for determining the blood sedimentation rate (ESR) according to the Westergren method.